Infographic
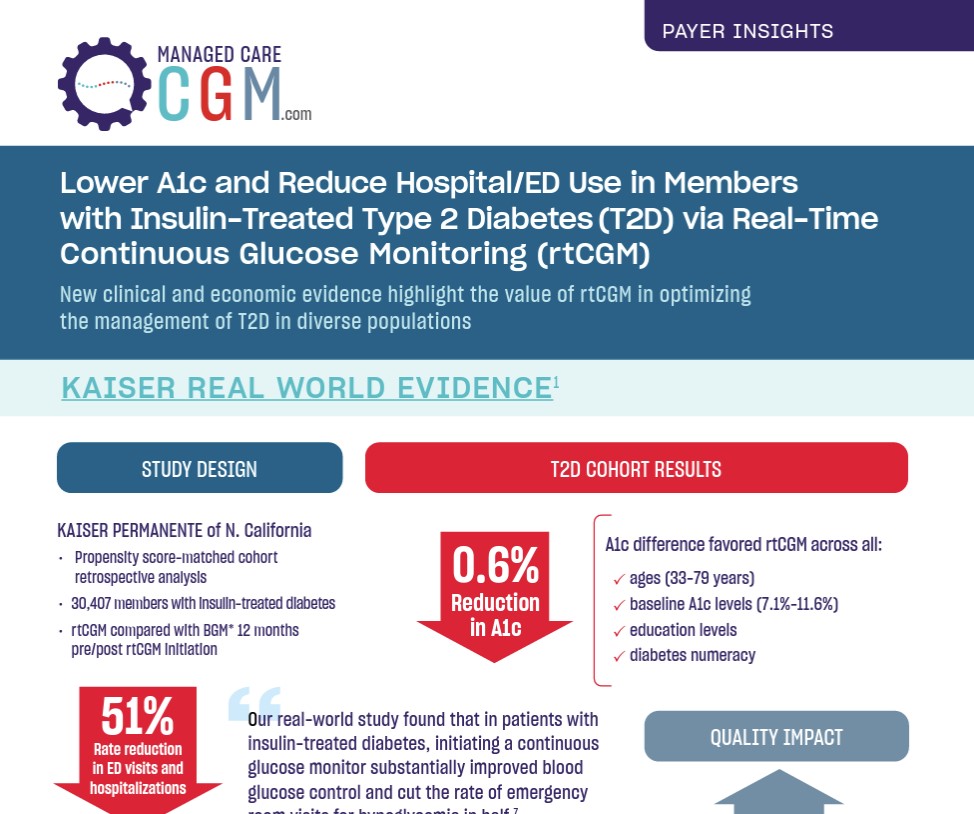
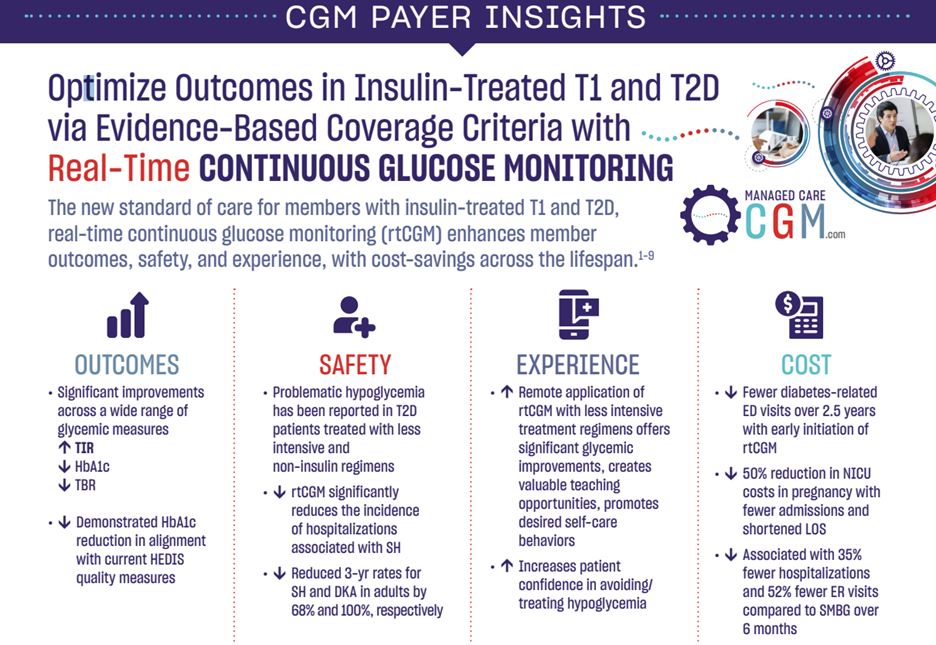
This interactive infographic highlights the clinical and economic value of CGM in the management of type 1 and type 2 diabetes (T1D and T2D) regardless of treatment regimen. The studies featured have been published in peer-reviewed journals and presented at the 84th American Diabetes Association (ADA) Scientific Sessions. Key areas of focus pertinent to payer professionals include long-term glycemic control in T2D, HbA1c reduction in managed care, improved T2D outcomes in the community setting, cost-effectiveness in T2D, and reduced hospitalizations in T2D. Collectively, this evidence demonstrates that appropriate coverage and utilization of CGM can improve clinical outcomes and reduce diabetes-related healthcare resource utilization. Summarizing the findings in a useful format, the infographic offers key takeaways for managed care and payer professionals associated with each individual study featured.
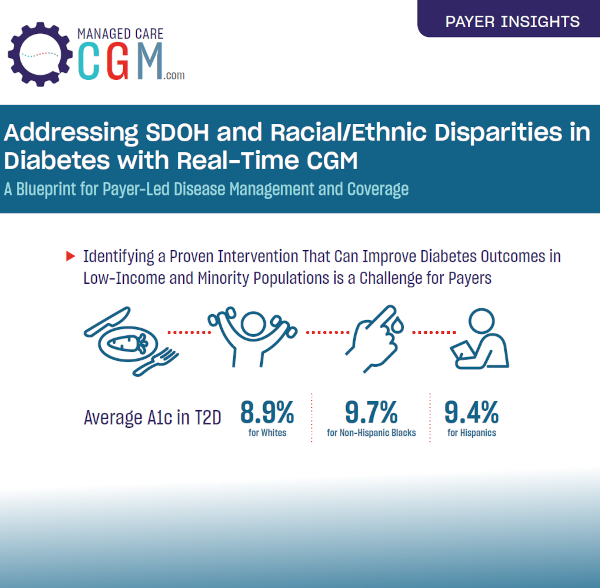
Learn MoreThis infographic highlights the value of CGM for positively impacting diabetes care quality, with special consideration given to underserved demographics of patients based on race/ethnicity and age. The piece links to the 2023 AMCP symposium moderated by Dr. Gary Puckrein, President and Chief Executive Officer of the National Minority Quality Forum, where diabetes care disparities and quality were also brought into focus. Also linked is an expert interview with Diana Isaacs, PharmD, with insights on disparities in diabetes care and quality considerations. Guidance is offered on improving CGM access as a means for meeting recent HEDIS quality measures tied to reducing emergency department and hospital use. Using findings from Blue Cross Blue Shield North Carolina as a case study, the infographic serves as a call-to-action for payers to remove manual prior authorizations for CGM under the pharmacy benefit.
- 1
- 1-8 of 8 results