Comparing Real-Time and Intermittently Scanned Continuous Glucose Monitoring in Adults with Type 1 Diabetes (ALERTT1): a 6-month, Prospective, Multicenter, Randomized Controlled Trial

Source: The Lancet – June 2021
Key Takeaways:
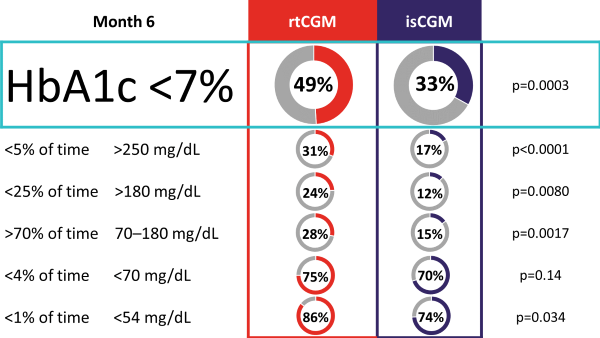
The ALERTT1 trial is the first 6-month, multicenter, prospective, randomized controlled trial comparing rtCGM with isCGM in 254 adults with type 1 diabetes, who previously used isCGM. Mean HbA1c was 7·4% (58 mmol/mol) and a minority of the study population was hypoglycemia unaware (44 [17%] people) or had a history of severe hypoglycemia (29 [11%]). Most (205 [81%]) were treated with multiple daily injections. Findings showed that in an unselected group of people with type 1 diabetes, 6-month use of rtCGM with alert functionality improved time in range (70–180 mg/dL [3.9–10.0 mmol/L]), while HbA1c, time in clinically significant hypoglycemia (< 54 mg/dL [3.0 mmol/L), and hyperglycemia (180 mg/dL [10.0 mmol/L]) were reduced. Additionally, more people on rtCGM achieved glycemic targets as defined by international consensus guidelines, and had less frequently severe hypoglycemia. Moreover, rtCGM users experienced less hypoglycemia worry and higher treatment satisfaction at the end of study.
Percentage of Participants Achieving Consensus Targets