Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults With Type 1 Diabetes
Source: Journal of the American Medical Association
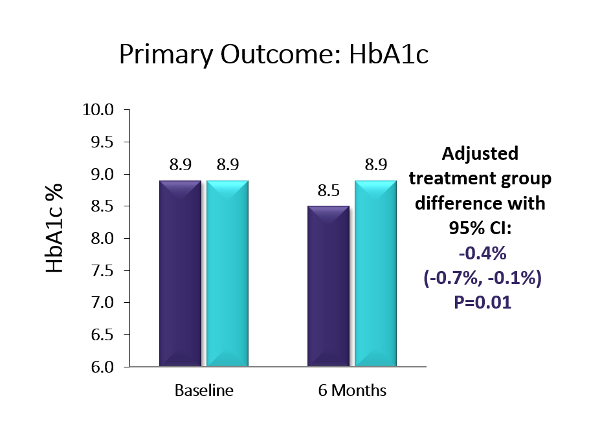
Key Takeaway: Six-month, multicenter, randomized controlled trial using the Dexcom G5. The baseline population had diverse racial/ethnic backgrounds (38% Hispanic or non-white), high baseline HbA1c levels, and 41% had public health insurance. The trial showed a 0.4% A1c advantage in favor of CGM over BGM (p=0.01; baseline: 8.9%). Moreover, more than twice as many in the CGM group as compared to the BGM group achieved an A1c reduction ≥0.5% (44% vs. 21%, p=0.005) and over four-times as many participants in the CGM group vs. the BGM group saw an A1c reduction of ≥1% (25% vs. 6%, p=0.003). The CGM group also saw a 1.7 hour/day advantage vs. BGM on time-in-range (70-180 mg/dl) (p<0.001). Over two-thirds of the CGM group were using CGM at least five days/week by the end of the six-month study – the highest CGM use observed for adolescents in a study to date. Moreover, the CGM group reported significantly higher glucose monitoring satisfaction, measured via the Glucose Monitoring Satisfaction Survey score, at 26 weeks than the BGM group . Newer models of CGM devices that eliminate fingerstick calibration should lead to improved wearability and glycemic control even beyond the measured benefits observed in this trial. Improved glycemic control early in diabetes duration may prevent diabetes complications later in adulthood, making CGM an attractive option for this population.