Author: admin
Source: The IQIVA Institute
About the report: The incorporation of time in range (TIR) metrics alongside HbA1c is expected to enhance the way in which diabetes is managed in the future, and subsequently, reduce the overall societal and economic burden. To assess the value of improving TIR from its current state to the minimum consensus target of 70% and 80% TIR, the IQVIA Core Diabetes Model was used to estimate cost reductions in complications and costs associated with improving TIR. Using this model, improvements in TIR were estimated to reduce the risk of developing diabetes-related complications resulting in a conservative reduction of $2.1-7 billion in costs over a 10-year period, based on the relationship between TIR and HbA1c. The addition of incrementally reducing hypoglycemic events in people with Type 1 Diabetes by 40% and improving TIR to 80% generated a total 10-year cost reduction of $6.7-9.7 billion. This reduction in costs represents a conservative estimate.
10-Year Cost Reduction by Improving TIR in People with T1 and T2 Diabetes to 70% and 80% TIR (US$Bn)
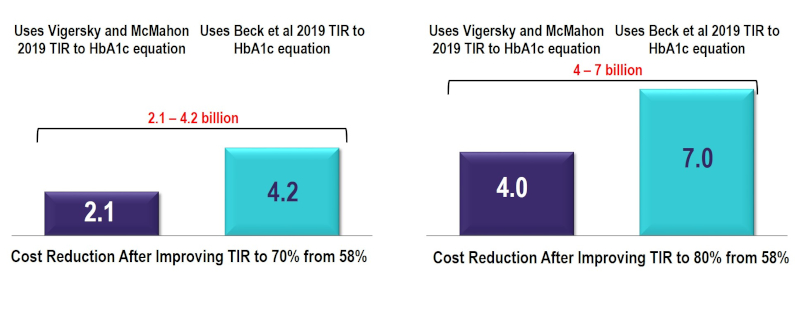
Source: IQVIA
Key Takeaway: This webinar presented the first estimation of reduction in complications and costs associated with improving time-in-range per research found in the Advancing Glycemic Management in People with Diabetes report. The slides presented are available to help you learn about the Time-in-Range movement and gain an understanding about what’s to come in the realm of diabetes care management.
LEARN MORESource: Centers for Medicare & Medicaid Services
Key Takeaway: CMS expanded Medicare coverage for therapeutic CGMs. Most notably, eliminating a requirement that beneficiaries use four fingerstick tests per day prior to accessing CGM. According to the local coverage determination, “there is no evidence to support that frequent SMBG (≥4 times per day) as a prerequisite for initiating CGM use is predictive of improved health outcomes”.
New Coverage Criteria (Effective July 18, 2021)
The revised LCD indicates that Medicare coverage for CGMs will be available if the beneficiary meets the following criteria:
- The beneficiary has diabetes mellitus; and,
- The beneficiary is insulin-treated with multiple (three or more) daily administrations of insulin or a continuous subcutaneous insulin infusion (CSII) pump; and,
- The beneficiary’s insulin treatment regimen requires frequent adjustment by the beneficiary on the basis of BGM or CGM testing results; and,
- Within six (6) months prior to ordering the CGM, the treating practitioner has an in-person visit with the beneficiary to evaluate their diabetes control and determined that criteria (1-3) above are met; and,
- Every six (6) months following the initial prescription of the CGM, the treating practitioner has an in-person visit with the beneficiary to assess adherence to their CGM regimen and diabetes treatment plan.
Source: Diabetes Technology and Therapeutics
Key Takeaway: There is growing and compelling evidence that CGM coverage should be offered to all patients who can benefit from this technology regardless of diabetes type and history of SMBG use. The current restrictions, which are based on outdated evidence and questionable assessments, are not supported in the literature. Moreover, they ignore the burden frequent SMBG places on individuals. Given the growing prevalence of diabetes, the persistent preponderance of individuals with suboptimal glycemic control, and the exorbitant and largely preventable cost of diabetes complications, opinion-based constraints should not continue to supplant evidence-based clinical management.
LEARN MOREIntended Audience: Case Managers – During the CCMC New World Symposium that took place from February 28, 2019, to March 2, 2019, Nicholas B. Argento, MD, FACE, Diabetes Technology Director of Maryland Endocrine in Columbia, Maryland, presented a satellite breakfast symposium on continuous glucose monitoring (CGM) that was sponsored by Dexcom.
LEARN MORESource: AMCP Science and Innovation Theater Webinar
Description: Dexcom G6® is the first CGM to receive the integrated CGM classification by the FDA. Dexcom G6® is approved for use as a standalone CGM and for integration with bluetooth connected insulin pens and automated insulin delivery systems. This presentation will highlight Dexcom’s technology, connected ecosystem, Google/Verily partnership, and future developments including EHR integration, population-level insights, and decision support algorithms.